New EU GMP Annex 1;Lyophilization and Contamination Control Strategy Should it be Your Concern?
News & Insights2022-12-26
As we all know the pharmaceutical industry is facing the new challenges of integrating the new EU GMP Annex 1 requirements into its current and new facilities, for some it will mean little change, for others there will be significant upheaval if they are to maintain their licenses and or facility approvals over the next few years.

This short article will begin to scratch the surface in some areas of what it may mean for some businesses and some of the key considerations and challenges that the new EU GMP Annex 1 presents.
So, let's take an initial look and the requirements in the new EU GMP Annex 1 for Contamination Control Strategy (CCS). The CCS is based on the Quality Risk Management (QRM) approach defined by the ICH (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use – for those who don’t know or were afraid to ask) Q9 which relates to risk management and a company’s own in-house CCS procedures.
All of these will be defined by the characteristics of sterile products and excipients being manufactured, the current status and design of equipment being used to manufacture the products and the current design of the manufacturing facilities.
Once it is established that the facilities and equipment are suitable for manufacturing with all control points identified and the process and facility are fully validated it is expected that a regular review process is undertaken as part of the CCS to ensure the control process is robust and consistent. This is where some businesses will have issues, firstly I suspect from the first review some will find that they do not have sufficient Contamination Control in place, while others who have taken this quite seriously for many years will find they have little to do.
The few paragraphs above only scratch the surface of what is required and if there is a knowledge gap in the business, this is where external expert knowledge and support is perhaps the best option ensuring your businesses do not have major audit failures or worse still product returns.
Let's look specifically at Lyophilization with respect to Contamination Control
The manufacturing and control of lyophilized products without doubt involve complex process technologies.
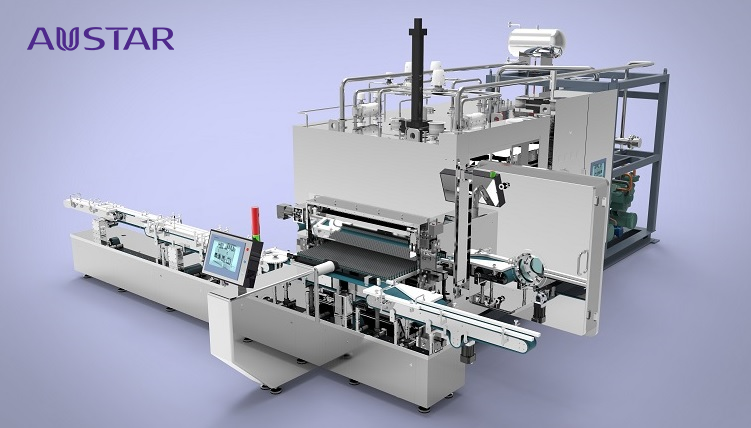
Every lyophilized product has its own unique properties and set of challenges however, in general, if I were going to look at this process from a top-down perspective these are some of the key elements I would begin with, and please note this if far from an exhaustive list, it very much the top level not a deep dive.
• Naturally the Product type, its potency, its density, it’s eutectic melting and/or vitrification breaking point, feeding time and temperature, freezing, sublimation, desorption, stoppering, discharging, vacuum degree desired, capping pressure and airflow volume within the chamber.
I think I would look at every transfer point, which is always a point of weakness, along with airflow studies and cleaning studies. I would even look at filter positions and materials of construction. Note that in some instances both cleaning and sterilizing agents can be absorbed into materials inside the manufacturing area which can affect the product.
• Obviously, the Product Container specifications (size and filling volume) and indeed materials, and this may or may not include any medical gases included in the manufacturing cycle. This may require further work with proficient and stringent audits of sub-suppliers.
• The Maximum ice load/condenser capacity, temperature, and surface areas

• Product freezing rate
• And lastly, Batch size (which affects the number of trays), handling and all manner of challenges
As stated previously this is not an exhaustive list, just some of the touch points to help you.
Lyophilization is a critical process step of the manufacturing of some sterile products and due to the process and equipment complexities along with process time consumption, the lyophilization procedure is likely to become the “bottleneck” in any sterile manufacturing cycle, and therefore in turn the sterility assurance and the CCS for lyophilization should be regarded as “critical” to the production process of sterile products.
What is CCS for Lyophilization?
Contamination Control Strategy (CCS) – That in this case can be defined in general as a planned set of controls for microorganisms, endotoxin/pyrogen, toxic materials, and particles are derived from the manufacturer's current product and process understanding. All of these, if validated and under regular review will prove and assure the process performance and product quality.
As stated previously other controls can include parameters and attributes related to any of the below:
•active substances
•excipients
•materials
•components
•facility and equipment operating conditions
•in-process controls
•finished product specifications, and the any associated methods and frequency of monitoring and control
Again, this is not an exhaustive list and will depend on your circumstances, a full audit of the operation certainly will help you with this and gain the business a much better understanding of their entire process.
What if you are Implementing CCS for Lyophilization?
The new EU GMP Annex 1 guidelines are not that different to other processes in respect to CCS, naturally there are some process specifics, but most of the guidelines could be and are intended to be general methodologies to update and improve GMP, which, if your process was installed correctly with due diligence being done around its validation then you are very likely to already be working virtually in line to the updated guidance.
However, let's look at some of the key points:
• The sterilization of the lyophilizer and associated equipment must be validated – you should not be operating if your process is not formally validated.
• The holding time between the sterilization cycle and use should be appropriately challenged during aseptic process simulation (APS) – this should form part of the original validation of the line and then repeatedly challenged at regular agreed and validated intervals.
• Lyophilizers and associated product transfer and loading/unloading areas should be designed to minimize operator intervention as far as possible – again many of the larger suppliers of this equipment are now using or developing robotic systems to remove this issue for manufacturers in areas such as loading and unloading.

• Adequate protection should be provided for the products from filling to lyophilization, such as using aseptic transfer, by selecting the appropriate barrier technologies – this is critical, and I would be careful with regards to Barrier Isolation to ensure that anyone supplying this equipment has suitable knowledge of this specialized technology. It is often the case that the manufacturers of the Lyophilization equipment think of Barrier Isolation as a simple “add-on” it is not, it can make or break a process in terms of sterility, handling of toxic products and operability. I would suggest that any business that is embarking on this for the first time I would suggest should seek external expert advice.

• The lyophilizer chambers and condensers should be thoroughly cleaned and sterilized, this will need looking at very carefully, some old Lyophilizers may be impossible to clean successfully due to the number of crevices, and even new models can be challenging.
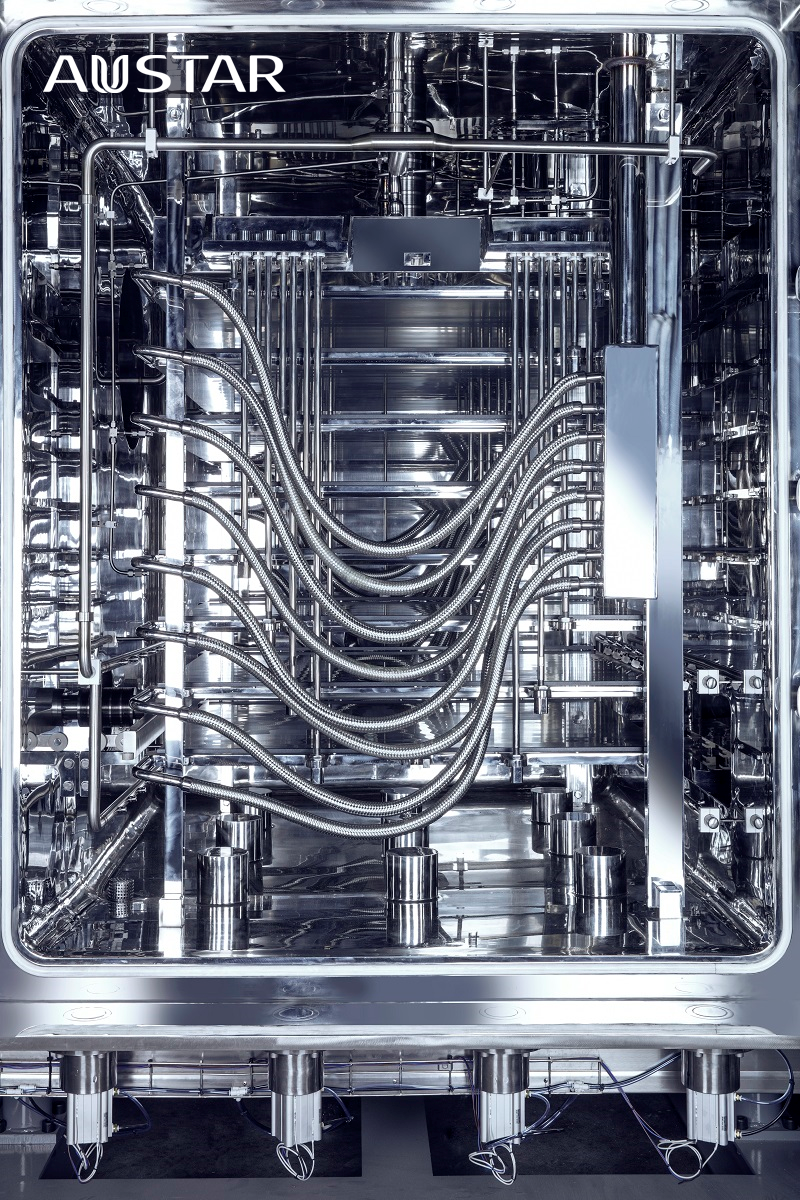
• The frequency at which a lyophilizer should undergo a full sterilization cycle, again something I would expect to be validated for each product being manufactured on each system as well as its design. Certainly, a validated sterilization cycle should be performed following any maintenance and or cleaning. All of these should be determined and based on the design and risks related to system contamination during use.
• If the system is fully automated I would also expect that the control system itself would form part of the Validation Package which would not just include the PLC software but any SCADA process management and or interfaces with both operators and equipment and or facilities/utilities associated with the process. I would also expect it to be validated in line with GAMP 5 concerning how the equipment is controlled both during the process and also during any automated sterilization or cleaning processes.
As discussed the new EU GMP Annex 1 has added some specifics on integrity under the section “Lyophilization” which include:
• Not just the sterilization Lyophilizer itself, but holding times between cycles.
• Specifically, manual loading and unloading should be sterilized between each load.
• The integrity of the Lyophilizer system should be maintained and even items such as the filter used should be sterilized before each use of the system with the results being part of the batch certification/release.
• Vacuum/leak integrity testing should be completed at the start of each cycle
• Other areas include :
o The transfer of Containers in Grade A Conditions
o As discussed previously Air Flow Patterns
o The use of Barrier Technology when dealing with partially stoppered vials
o Ensuring that the product that is open remains under Grade A conditions if removed from the Lyophilizer
o Utensils used during transfer, loading and unloading (including Trays, bags, placing devices, tweezers etc.) should also be subject to a validated sterile process.
In essence, the lyophilization process should be developed early in the product development cycle due to its significant impact on the capacity and the cost of the facilities used for the manufacturing of sterile products.
Conclusion
This short article is intended to give you a starting point in the process and to gain an understanding of the implications of some of the aspects of the new EU GMP Annex 1 guidance about CCS and lyophilization.
If you have had little experience of this process or indeed the application of the new guidance, EU GMP Annex 1, the best way to ensure the security of your business is always to seek expert guidance.
This article certainly does not cover all aspects but hopefully will point you in the right direction and to the right people.
For further information or guidance on this and or other aspects please contact one of our team who will help you with your process.
Ewart Richardson AUSTAR Consultant
info@austar.com.hk
https://www.austarunion.com/







 Search
Search 中文
中文











